Expressing This RNA Can Silence An Entire Chromosome
We carry a pair of each autosome but what about the sex chromosomes? In females, they occur in pairs (XX) whereas in males, X is…
We carry a pair of each autosome but what about the sex chromosomes? In females, they occur in pairs (XX) whereas in males, X is accompanied by Y. Consequently, a X-linked gene that is expressed in males once, will be expressed twice in females thus generating a monosomy-like condition in males (or trisomy-like condition in females).
Although trisomy of some gene-poor chromosomes is tolerated and manifests in the form of severe diseases (Down syndrome occurs due to the trisomy of chromosome 21), the monosomy of autosomes is lethal and leads to miscarriages. Still, despite the monosomy-like condition of X-Chromosome, males turn out to be perfectly normal.
So, our cells must be designed in a way that it needs only one dose of X-linked genes. But then, the XX (female) cells enter into the problem of producing twice the required dose. Yet even the females turn out to be perfectly normal. This suggests that there exists a dosage compensation mechanism, either in males or in females.
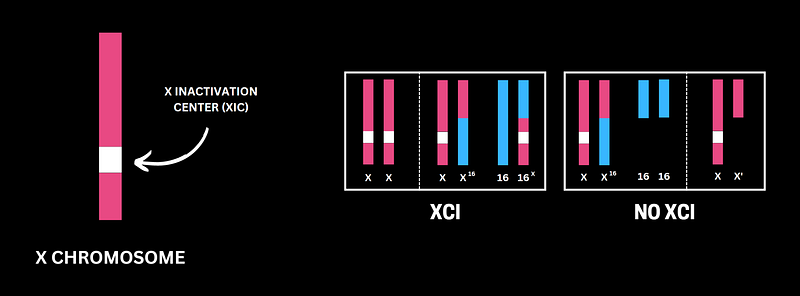
Although different dosage compensation mechanisms are seen in different organisms, Mammals generally solve this problem by silencing an entire X chromosome in the XX cells — a phenomenon which is called X-Chromosome Inactivation (XCI). This remarkable feat of silencing not one gene but an entire chromosome is achieved by a cockpit (called XIC) present in the X-Chromosome in which sits the pilot — called Xist.
The Cockpit And The Pilot
Experimental observation of in vitro cells containing truncated and translocated X chromosomes lead to the identification of XIC — X-Inactivation centre. XIC is a region present on the X chromosome that is both necessary and sufficient to trigger X-Chromosome Inactivation. But a cell always needs two XICs two initiate the process of X inactivation
XIC is like a cockpit that houses a range of elements (the crew) that is necessary for the inactivation process along with a pilot — The Xist (X-Inactivation Specific Transcript) — that drives the process of X-Inactivation.
The Xist gene encodes a long non-coding RNA (lncRNA) called Xist. When synthesized, Xist spreads in both directions, eventually coating the X chromosome from which it was produced. This RNA then recruits several proteins that act on the chromosome to modify and condense its structure. What remains is a highly condensed structure, devoid of transcriptional machinery, which is called Barr Body.
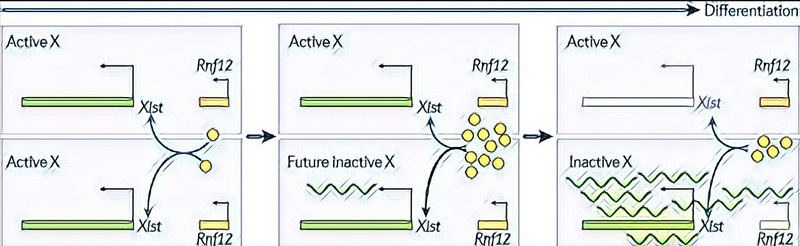
In the female (XX) cells, this Xist is expressed on only one of the chromosomes and is not expressed on the single X chromosome of the male cells. The other X chromosome of females and the only X chromosome in male expresses Tsix — the antisense transcription unit of Xist. This Tsix prevents the expression of Xist and keeps the chromosome in the active state.
How does the cell choose the chromosome that will express Xist and be inactivated? It turns out that the choice is random. In the stem cells, both X-chromosome express Xist at very low levels. But upon differentiation, Xist is upregulated on any one chromosome which is then inactivated. The other chromosome expresses Tsix which ensures its active form.
Some cells inactivate the paternal X whereas some inactivate the maternal one. As a reason, females are mosaic in terms of X-Chromosome, containing both types of cells — some with iXp (inactivated paternal X-chromosome) and some with iXm (inactivated maternal X-Chromosome).
Some pluripotency factors have been identified that binds to the Xist gene and inhibits its transcription in the stem cells. Upon deletion, these factors have resulted in upregulation of Xist. Although, even after the deletion of the binding site of these factors on Xist gene, its transcription showed no effect, suggesting that additional factors and mechanisms exist to repress Xist.
A Remarkable Counting Precision
So, one X is inactivated in females and males retains its only X in the active form. What if there is a trisomy of X (this time, I’m talking about real trisomy, that is XXX cells)? In this case, two of the X chromosomes are inactivated. Similarly, three of X chromosomes are inactivated in XXXX cells. And in case of monosomy of X (XO cells), the single X escapes inactivation.
Consequently, each cell has just one active X chromosome. To achieve this remarkable precision, there must exist some sort of counting mechanism. What is that? How does a cell count the number of X-Chromosomes and inactivate all except one? Although several models have been proposed, none has been proved.
One of the models suggests the existence of a negative feedback loop involving trans-acting factors like RNF12 which can activate Xist. The Rnf12 gene, again located in the cockpit, synthesizes this factor. RNF12 can activate one of the Xist only if it is present in an amount more than a certain threshold value. It is proposed that this value is reached only if both the X-Chromosome synthesizes RNF12. Thus, initially both X are active and synthesize RNF12 which then acts to activate any one Xist. Upon activation of one Xist, the corresponding X is inactivated, causing a stop in the expression of all X-linked genes including Rnf12. Thus, the Rnf12 level drops to half — such a low amount fails to activate the other Xist, sparing that chromosome from inactivation.
It has been shown that the overexpression of the only Rnf12 in males can induce Xist coating of the single X-Chromosome. But when the Rnf12 gene was deleted from one X-Chromosome in females, it did delay the inactivation process but did not impede the same. This suggests that several such activating (and may be repressing) factors can act in cis or trans to ensure the correct counting of X-Chromosome.
Another proposed model advocates for stochastic XCI. This model basically discards the need of any counting mechanism by suggesting that X inactivation can occur in any fashion in different cells. Some cells can have both X in active state whereas some can have both in inactive state. And some of the cells will carry out XCI correctly, possessing one active and one inactive X. XCI is then followed by selection in which the cells showing correct XCI are selected and allowed to proliferate. Cells showing incorrect XCI either dies (as both incorrect XCI proves to be fatal) or correct its XCI pattern and then undergoes proliferation.
Apart from these models, some studies involving triploid and tetraploid cells suggest the involvement of autosomes. It has been shown that cells do not just maintain one active X chromosome, but they maintain one active X per diploid set of autosomes. Also, for the same number of X chromosomes, XCI occurs rapidly in cells with high X/A ratio (where X is the count of X-Chromosome and A is the count of Autosomes). This fact strengthens the blocking factor hypothesis which is quite similar to the model based on Rnf12 factor.
According to the blocking factor hypothesis, a factor synthesized by autosomes binds to and blocks the synthesis of Xist. But the autosomes synthesize it in an amount enough to bind to only one Xist allele, rendering the other Xist allele unbound. Hence, one of the X (with blocking factor bound to Xist) remains active whereas other X chromosomes are inactivated. But till date, no such factor has been identified.
What could be the nature of such autosomal factors? As previously mentioned, pluripotency factors have repressing roles in the synthesis of Xist. Since most of the pluripotency factors are autosomal, such factors can take part in this mechanism of counting.
The Takeaway
To deal with the genetic imbalance, placental mammals evolved a dosage compensation mechanism in which they inactivate extra X chromosomes. But other organisms have different mechanisms such as in flies, dosage compensation is carried out in male by upregulating the single X chromosome approximately twofold.
In mammals, the process of X inactivation (XCI) is carried out by Xist that is located in XIC (X-Inactivation Centre). XCI is not a simple process. It is a complicated process that is under control of not one but several elements. Even the Xist is controlled by many factors including RNF12, Tsix and pluripotency factors (like NANOG, OCT3/4, etc.). Similarly, Tsix is also regulated by range of factors. Deletion of one or two factors does impact the XCI but does not completely stop the process suggesting that the system houses several backups and alternative routes.
The choice of which chromosome should be inactivated is completely random. But intervening the XIC (and thus XCI) has caused skewing of this choice. Like when Tsix is deleted on one chromosome, that chromosome is inactivated more frequently than the other, wild type X-Chromosome. And on contrast to the random X-Inactivation, Marsupials inactivates only the paternal X-Chromosome.
Given the complexity of the system and process, although several models have been proposed for counting, but none has been proved. Existence of a simple counting mechanism seems difficult. There must be a complicated network of elements, factors and regulators that would be involved to ensure the precision of counting. Such networks remain to be discovered.
References :
X-chromosome inactivation: counting, choice and initiation - Nature Reviews Genetics
In many sexually dimorphic species, a mechanism is required to ensure equivalent levels of gene expression from the sex…www.nature.com
Regulation of X-chromosome inactivation by the X-inactivation centre - Nature Reviews Genetics
Recent studies have greatly increased our understanding of the molecular actors that regulate X-chromosome inactivation…www.nature.com